Nagoya, Japan
September 20, 2017
Scientists at ITbM, Nagoya University have synthesized a new bioactive small molecule that has the ability to increase stomata numbers on flowering plants without stunting their growth. The team's new discovery could help elucidate the stomatal development mechanism in plants.
Environmental studies have shown that 40% of the atmospheric carbon dioxide (CO2) passes through plant stomata every year. Thus, controlling stomatal development and function is considered as a key for increasing crop plant productivity and water-use efficiency. Stomata are pores found in plant leaves that are responsible for gas exchange with the surrounding environment. As it has been reported that light and atmospheric CO2 levels influence the number of stomata, synthetic chemists and plant biologists at the Institute of Transformative Bio-Molecules (ITbM) in Nagoya University, have decided to explore this topic using a chemical approach and succeeded in developing small molecules to increase the number of stomata in plant leaves. The result of this study was reported in the journal, Chemical Communications.
Using a model flowering plant, Arabidopsis thaliana, ITbM's research group performed a chemical screening of selected small molecules discovered from ITbM's chemical library and identified two molecules (CL1 and CL2) with a similar structure to the non-steroidal anti-inflammatory drug, Celecoxib©. Although CL1 and CL2 increased the number of stomata in plant leaves, they were toxic to the plants when applied at high concentrations.
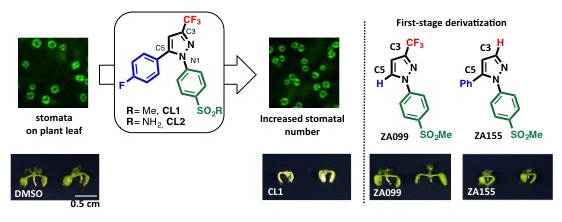 Figure 1. Compounds CL1 and CL2 increase the number of stomata on plants, although they inhibit plant growth (left). Compound ZA155 (right) increased the number of stomata, but also inhibited plant growth. Compound ZA099 increased the number of stomata and had no effect on plant growth inhibition.
Figure 1. Compounds CL1 and CL2 increase the number of stomata on plants, although they inhibit plant growth (left). Compound ZA155 (right) increased the number of stomata, but also inhibited plant growth. Compound ZA099 increased the number of stomata and had no effect on plant growth inhibition.
Encouraged by the plant stomata increasing effect of CL1 and CL2, the team designed the structure of the molecules to develop new compounds that can increase the numbers of stomata, while minimizing the toxicity upon exposure of the plant to the compounds at high concentrations. The team synthesized and tested small molecules absent of the trifluoromethyl (CF3) group in the C3-position (ZA155) or the aryl group in the C5-position (ZA099) on the pyrazole (a 5-membered heterocycle consisting of three carbon atoms and two adjacent nitrogen atoms) ring. As a result, the team discovered that although both compounds led to an increase in stomatal number, ZA155 led to growth inhibition of the plant, while ZA099 did not.
"I started this research when I arrived at ITbM in 2015," says Dr. Asraa Ziadi, a postdoctoral researcher at ITbM who mainly synthesized the molecules. "With my background in organometallic chemistry, I wanted to do something different but still use my expertise."

The synthetic chemistry team was led by Professor Kenichiro Itami (pictured above), the center director of ITbM, and they developed a rapid palladium-catalyzed C-H arylation methodology that would allow the direct synthesis of a range of aryl pyrazole derivatives from ZA099 and their corresponding aryl bromides, in hope of increasing the number of stomata while avoiding growth inhibition. By using their new synthetic method, they were able to directly replace the hydrogen (H) atom connected to the carbon (C) atom on the pyrazole ring with various aromatic rings (C-H functionalization) in order to conduct structure activity relationship studies.
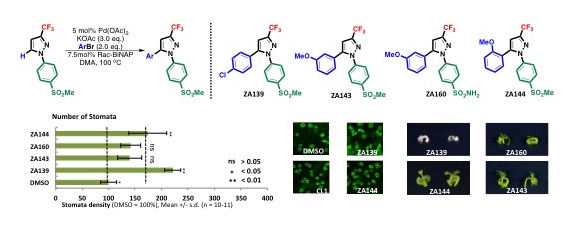 Figure 2. Palladium-catalyzed C-H arylation towards the synthesis of aryl pyrazoles. These new compounds have an effect on the number of stomata in plants.
Figure 2. Palladium-catalyzed C-H arylation towards the synthesis of aryl pyrazoles. These new compounds have an effect on the number of stomata in plants.
Upon investigating the effect of the synthesized small molecules on plant stomatal numbers, it was observed that a chlorine-containing compound (ZA139) generated high stomatal density on leaves, but was extremely toxic to the plant, leading to an abnormal stomatal shape. As the methoxy-containing ZA143 led to a small increase in stomatal numbers and was not severely toxic to the plant, the group thought that perhaps the sulfonamide analogue ZA160 would perform better. The compound, however, did not increase the number of stomata on plant leaves and led to growth inhibition.
Next, the team turned their attention towards synthesizing and testing different anisole (methoxybenzene) substituted compounds that could increase the number of stomata without inhibiting plant growth. Indeed, they were able to identify ortho-anisyl substituted ZA144, which has the methoxy group in the ortho-position, as the most effective molecule in increasing the number of stomata without severe toxicity.
"The best moment of this research was setting up the biological experiment and seeing the increase in the number of stomata on the plant leaves under the microscope," describes Ziadi. "I remember thinking "my molecules did that!"; this was a great feeling."

Biological experiments on plants were conducted by a group of plant biologists, led by Professor Keiko Torii (pictured above), a principal investigator at ITbM who also holds a position at the University of Washington. Ziadi has worked closely with plant biologist Naoyuki Uchida, who is an associate professor in Professor Torii's group, and speaks about the challenges of carrying out biological research as a chemist.
"For me, it was understanding the biology behind the discovery," says Ziadi. "As a synthetic chemist, your role usually ends when the molecule has been synthesized. But at ITbM, you get to see what the molecules can do. That's really interesting! I was very intrigued by the idea of synthesizing molecules that can give such visual and clear changes in plant."
"I was always surprised that, every time I told Asraa about the effects of the molecules she synthesized on stomata number and plant growth, she started synthesizing more molecules with better effects on the same day," describes Uchida. "This amazingly speedy collaboration between biologists and chemists was possible only in a research environment like our institute, where biologists and chemists work together next to each other. We enjoy this collaboration so much."
The key to the group's success in identifying a small molecule that can enhance the number of plant stomata was the development of a C-H functionalization reaction by synthetic chemists that enables rapid derivatization of aromatic rings. This accelerated plant biology research to access a series of bioactive molecules, which induces desirable stomatal development without inhibiting plant growth.
Further investigations using their bioactive pyrazole compounds may lead to the clarification of the mechanism behind pyrazole-mediated stomatal differentiation. This may lead to possible identification and synthesis of compounds that can increase biomass through stomatal control.
"I learned that collaboration between biologists and chemists is very powerful," says Ziadi. "You can learn so many things and discuss the project from different aspects. In my case, to understand better the project, I started to educate myself on stomata and the different mechanisms that can be involved in stomatal development. It was difficult, but luckily, I am in an institute where I'm surrounded by excellent researchers from different disciplines."
Journal Information:
"Discovery of synthetic small molecules that enhance the number of stomata: C-H functionalization chemistry for plant biology" by Asraa Ziadi, Naoyuki Uchida, Hiroe Kato, Rina Hisamatsu, Ayato Sato, Shinya Hagihara, Kenichiro Itami and Keiko U. Torii is published online in Chemical Communications.
DOI: 10.1039/C7CC04526C