Elkhart, Indiana, USA
June 20, 2019
Agdia, Inc. has commercialized a rapid and user-friendly, DNA-based assay, on their AmplifyRP® XRT platform, for the detection and identification of Clavibacter michiganensis subsp. nebraskensis (Cmn).

The bacteria Clavibacter michiganensis subsp. nebraskensis is the causal agent of Goss’s bacterial wilt and leaf blight on susceptible dent corn, sweet corn, and popcorn varieties. This pathogen survives primarily on infested crop residues that serve as primary inoculum; however, seed-to- seedling transmission of Cmn has been observed at low rates. The Cmn bacteria is an incessant and economically important pathogen of corn, and severe losses occur due to leaf blight and vascular wilt. Symptoms of Goss’s Wilt (Figure 1.) can be mistaken for leaf scorch due to water stress, wind desiccation, chemical burn, or a second bacterial disease known as Stewart’s wilt.
The AmplifyRP® XRT assay for Cmn is an isothermal amplification technology that implements the recombinase polymerase amplification (RPA) platform. This method promotes the rapid amplification and detection of nucleic acid targets, DNA or RNA, via enzymatic reactions, and maintains a single operating temperature of 39o C. The RPA technology provides comparable sensitivity and specificity to polymerase chain reaction (PCR) assays while offering advantages to PCR. Crude plant extracts are processed for RPA, and total assay time is approximately 30 minutes, including sample preparation. Moreover, RPA does not require expertise in molecular diagnostics to perform. For more information on Agdia’s complete line of AmplifyRP® assays, please visit Agdia’s website www.agdia.com.
Agdia validated their new assay against a collection of 36 known isolates of Cmn, all of which were detected and identified consistently, during their analysis. Moreover, no cross-reactivity was observed with several species of plant pathogenic bacteria, including closely related subspecies of Clavibacter michiganensis. Agdia states their RPA assay for Cmn detection is comparable or superior to all available molecular and serological diagnostics, in sensitivity and specificity.
To perform the assay, the end-user initially extracts their sample using a mesh extraction bag and buffer. After the sample has been extracted, a small volume of sample is added to a solution that is used to rehydrate a lyophilized reaction pellet. Once the reaction pellet has been rehydrated, it is added to a portable fluorometer and heated at 39o C for 25 minutes. During this time, the target Cmn DNA, if present, will be amplified and produce a fluorescent signal that is detected by the fluorometer in real-time and visualized as an amplification curve.
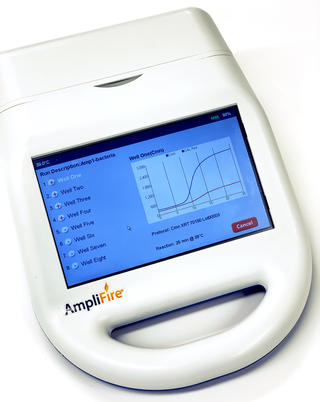
Agdia recommends using the AmpliFire® portable fluorometer (picture above) for use with their AmplifyRP® XRT and XRT+ products. The AmpliFire® is battery-operated and portable,
allowing it to be implemented in remote locations. The initial programming set up on the AmpliFire® is simple via pre-developed barcoding included with product purchase; optimal assay protocol parameters are determined during development. A large, intuitive touchscreen makes workflow simple and straightforward while data export options provide for detailed interface with personal computers. For more information on the AmpliFire® portable fluormeter, please visit Agdia’s website.
About Agdia
A leading provider of diagnostic solutions for agriculture, Agdia, Inc. has been serving plant breeders, propagators, growers, universities, and private testing laboratories since 1981. The company offers a comprehensive portfolio of validated, easy-to-use diagnostics for identifying plant pathogens, plant hormones, and transgenic traits. Moreover, Agdia operates an ISO accredited, in-house, testing services laboratory. Agdia’s quality management system is ISO 9001:2015 certified and their Testing Services Laboratory is ISO 17025:2005 accredited. Visit the company’s website at www.agdia.com, e-mail info@agdia.com, phone 1-574-264-2615 (toll-free 800-622-4342) or fax 1-574-264-2153.
About AmplifyRP®
AmplifyRP XRT Test Kits employ recombinase polymerase amplification (RPA) technology licensed by Agdia, Inc (licensee) from TwistDx Limited, U.K (licensor). Use of the RPA process and probe technologies are protected by US patents 7,270,981 B2, 7,399,590 B2, 7,435,561 B2, 7,485,428 B2 and foreign equivalents in addition to pending patents.
AmplifyRP® and AmpliFire® are registered trademarks of Agdia, Inc.